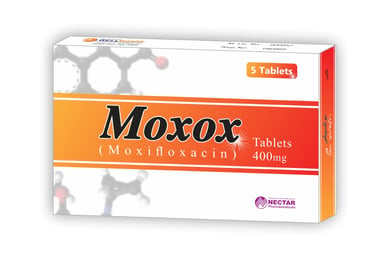
Welcome to Wellborne Pharmachem & Biologicals
Ensuring Quality to Ensure Efficacy
"Born to heal, shaping a healthier tomorrow"
About Wellborne
Wellborne Pharmachem & Biologicals is an ISO-certified pharmaceutical company, operating since 2009 with a state-of-the-art cGMP-compliant facility in Industrial Estate Hattar, near Islamabad.
We manufacture high-quality medicines across diverse therapeutic areas, backed by skilled professionals, modern equipment, and validated systems. Our operations meet the highest international standards for safety, efficacy, and quality control. With a global vision and commitment to continuous improvement, Wellborne is dedicated to advancing healthcare worldwide.





To produce quality medicines that conform to cGMP requirements and cater to the healthcare needs of both local and international markets.
To employ cutting-edge technologies that are environmentally friendly, striving to achieve zero-defect products by meeting international standards.
To work tirelessly to provide the highest quality and affordable medicines to the ailing community, thereby contributing to the betterment of society.
Our Mission
To become a leading pharmaceutical company in Pakistan, serving the nation’s ailing community with quality and affordable medicines, and extending our efforts to promote good health globally.
Our Vision













Departments & Divisions
Supply Chain
Our Supply Chain Department manages sourcing, procurement, and secure distribution of medicines in compliance with regulations and global standards, ensuring integrity, quality, safety, and timely availability.


Quality Assurance
Our Production Department is dedicated to delivering high-quality medicines by upholding the principles of cGMP compliance and leveraging Six Sigma methodologies to eliminate defects, and foster a culture of continuous improvement and operational excellence.
Our Quality Control (QC) Department ensures our products meet safety, purity, and potency standards by following Good Laboratory Practices (GLP), FDA, WHO, ICH, and Pharmacopoeia guidelines through rigorous testing and documentation.
Production






Quality Control
Our Quality Assurance (QA) Department ensures our products meet global standards by following cGMP and complying with FDA, WHO, and ICH guidelines, safeguarding patient health and product integrity.
Wellborne operates through specialized departments and divisions, each driving excellence across manufacturing, quality, supply, and innovation.

Contact Us
Reach out for inquiries about our high-quality medicines and services.
Quick Links
info@wellbornepharma.com
+92 333 0240 000
+92 995 617333
© 2025. Wellborne Pharmachem & Biologicals. All rights reserved.
Let’s Connect @
Contact Us
Designed & Developed by Globex Solutions